Study on hygroscopicity of polyacrylamide and its porous materials
1 Introduction Natural Green Hair Crystal Single Circle Bracelet, Natural Green Rutilated Quartz,Green Hair Crystal Faceted Gemstones Guangzhou Qinfa Crafts Company , https://www.qinfastone.com
Organic polymer moisture-absorbing materials are a new type of functional polymer that was initially developed from superabsorbent resins. These materials have remarkable moisture absorption and retention properties. They are water-based resins modified through chemical and physical methods to absorb water via hydrophilic groups in their molecular structure. Compared to traditional inorganic hygroscopic materials, organic polymers exhibit higher moisture absorption capacity, often exceeding 70%, with a rapid absorption rate of up to 2 mg/g·min. Additionally, they do not cause corrosion, pollution, or require regeneration [1–3]. The versatility of these materials—available as powders, granules, strips, or transparent films—makes them suitable for various applications, meeting diverse moisture control needs.
Polyacrylamide (PAM) shares similar side groups—amide groups—with acrylamide units along its chain. These amide groups readily form hydrogen bonds with water or substances containing -OH groups, such as natural fibers, proteins, soils, and minerals, leading to strong adsorption. Due to this high hygroscopicity, PAM is widely used as an organic moisture-absorbing material. However, current hygroscopic materials, both domestically and internationally, face challenges such as limited moisture absorption performance and poor reusability, with little systematic research on organic polymer-based materials. This paper investigates the effects of various polymerization conditions on PAM's hygroscopic properties, aiming to optimize the process for faster, more efficient moisture absorption without corrosion or environmental impact.
2 Test Methods and Experimental Plans
2.1 Raw Materials
Acrylamide (AM), AR grade, from Beijing Yili Fine Chemicals Co., Ltd.; sodium hydroxide, AR grade, from Beijing Beihua Fine Chemicals Co., Ltd.; sodium sulfite, AR grade, from Beijing Yili Fine Chemicals Co., Ltd.; ammonium persulfate, AR grade, from Beijing Chemical Plant; N,N’-methylenebisacrylamide (MBAA), AR grade, from Beijing Hongxing Jinxing Chemical Factory; color-changing silica gel (Type A), from Qingdao Ocean Chemical Co., Ltd.; molecular sieve (3A), from Dalian Haixin Chemical Co., Ltd.; polyethylene glycol PEG200, AR grade, from Shanghai Pudong Gaonan Chemical Plant; calcium carbonate (CaCO3), AR grade, from Beijing Red Star Chemical Plant; and deionized water were used in the experiments.
2.2 Preparation of Polymers
2.2.1 PAM Preparation
The monomer AM was weighed and dissolved in a beaker, then mixed evenly. The crosslinker MBAA and the initiator [Na2SO4-(NH4)2S2O8] were added, followed by pH adjustment using a NaOH solution to neutralize the mixture. The total weight was measured, and deionized water was added to reach the desired volume. The mixture was purged with nitrogen and placed in a constant-temperature water bath for reaction.
2.2.2 PAM/PEG Preparation
Similar to the above process, PEG was added after adjusting the pH to neutral. The mixture was heated in warm water for 30 minutes and then cured at 60°C for 4 hours in an oven.
2.2.3 PAM/CaCO3 Preparation
After the same initial steps, CaCO3 was introduced as a porogen. The final product was washed thoroughly with hydrochloric acid and distilled water to remove excess acid and obtain the final sample.
The resulting gels were washed, sliced, dried at 120°C, ground, and sieved to prepare the samples.
2.3 Moisture Absorption Performance Test
Ten grams of each sample were dried in an oven and weighed to 0.001 g under controlled conditions (≤5 s, ≤50% RH). The samples were then placed in a humidity chamber for moisture absorption testing. Weighing occurred every hour until two consecutive weighings differed by less than 5 mg. The moisture absorption rate was calculated using the formula provided below: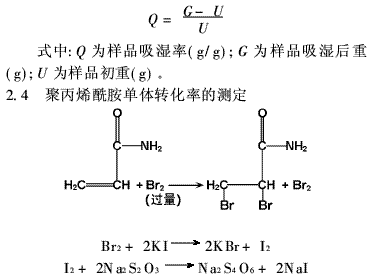
2.4 Determination of Monomer Conversion Rate
2.4.1 Bromine Solution Preparation
10g KBr and 2.79g KBrO3 were dissolved in 1000ml volumetric flask and diluted to the mark.
2.4.2 Experimental Procedure
A 0.05g PAM sample was placed in a 250ml conical flask, and distilled water was added. Then, 20ml bromine solution was added from a burette, followed by 4–5ml of 3mol/L sulfuric acid. The mixture was shaken well and left in the dark for 2–3 hours. Next, 10ml of 20% potassium iodide solution was added, and the mixture was titrated with 0.05mol/L sodium thiosulfate until the solution turned light yellow. Finally, 0.2ml of 0.5% starch solution was added, and titration continued until the blue color disappeared. A blank test was also conducted. The monomer conversion rate was calculated as follows:
The conversion rate of PAM was found to be over 75%.
3 Results and Discussion
3.1 Mechanism of Moisture Absorption
As shown in Figure 1, PAM absorbs moisture faster than molecular sieves and silica gel. After 150 minutes, the hygroscopic curves of silica gel and molecular sieves plateau, indicating that they have reached their maximum moisture absorption capacity, which is relatively low. This is due to their non-elastic, porous structures, which allow for strong surface adsorption but limited internal penetration. Silica gel contains Si-O bonds that react with water molecules upon exposure to humid air, forming chemisorbed water. However, since it does not swell, its overall moisture absorption capacity remains limited.
In contrast, PAM exhibits both physical and chemical adsorption. While the initial physical adsorption is fast, the slower chemical adsorption allows for deeper moisture penetration into the polymer network, significantly enhancing its hygroscopic performance. According to the “three-state water model,†water can exist as free, bound, or physically adsorbed water, depending on its interaction with the polymer’s functional groups. PAM’s high water absorption ratio far exceeds that of traditional materials like silica gel and molecular sieves, giving it a much greater moisture absorption capacity.
3.2 Effect of Reaction Conditions on Hygroscopic Performance
3.2.1 Effect of Monomer Concentration
The hygroscopic properties of PAM prepared at different monomer concentrations vary significantly. At 20–26% concentration, the moisture absorption rate increases with concentration, peaking at 26%. Beyond this, the rate declines, likely due to changes in crosslinking density and molecular weight.
3.2.2 Effect of Crosslinking Agent
When the crosslinking agent (MBAA) content is 0.12%, PAM shows the highest moisture absorption rate. Increasing the amount beyond this point reduces the rate, likely due to increased crosslinking density and smaller pore sizes.
3.2.3 Effect of Urea Content
The addition of urea affects the crosslinking process, reducing the formation of poorly soluble materials. However, excessive urea lowers the molecular weight, decreasing the number of effective moisture-absorbing groups and thus reducing the absorption rate.
3.2.4 Effect of Degree of Hydrolysis
The degree of hydrolysis influences the pH of the solution, which in turn affects the moisture absorption rate. Higher hydrolysis levels increase the pH, promoting better moisture uptake. However, the alkaline hydrolysis of PAM leads to self-blocking effects, slowing the reaction rate.
3.2.5 Effect of Polymerization Temperature
The optimal temperature for PAM synthesis is around 50°C. Both too high and too low temperatures negatively affect the material’s properties, based on reaction kinetics.
3.2.6 Effect of Porogen
The use of PEG as a porogen creates uniform macropores on the PAM surface, greatly increasing its specific surface area and moisture absorption performance. In contrast, CaCO3 produces only sparse mesopores, resulting in less effective moisture absorption.
4 TGA Analysis
Thermal gravimetric analysis (TGA) showed that PAM/PEG has slightly lower thermal stability compared to pure PAM, while PAM/CaCO3 exhibits improved thermal resistance.
5 Conclusion
The specific surface area plays a critical role in determining moisture absorption performance. The addition of PEG as a porogen significantly enhances the hygroscopic performance of PAM by creating uniform pores. However, the effect of CaCO3 as a porogen is minimal, resulting in less effective moisture absorption. Under test conditions (20°C, 90% RH), PAM outperformed conventional silica gel and molecular sieves in both moisture absorption capacity and rate, highlighting the advantages of organic polymer-based moisture absorbers. TGA results confirmed that PAM/PEG has slightly reduced thermal stability, while PAM/CaCO3 shows improved thermal resistance.